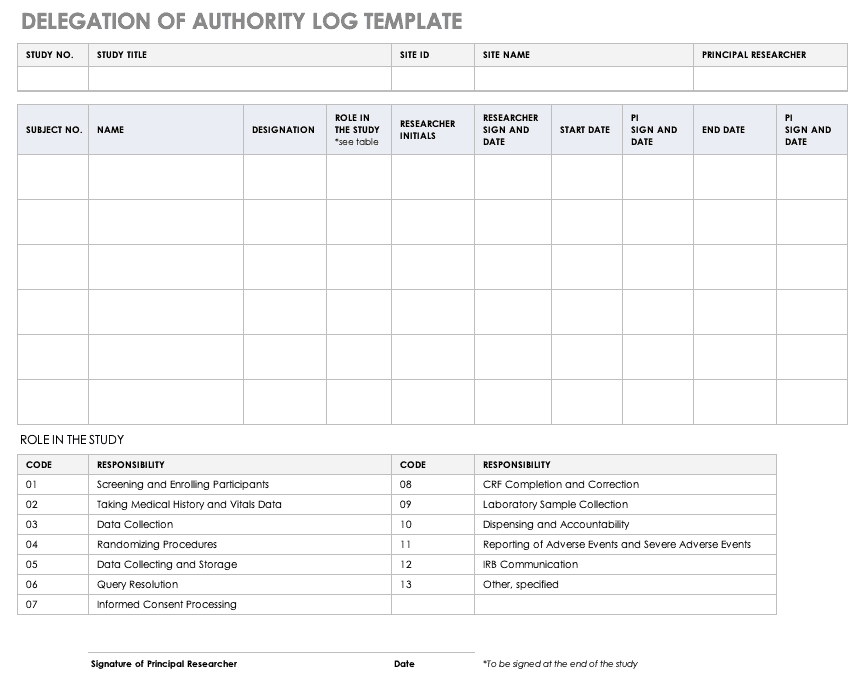
Case Report Form Template Clinical Trials (4) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Templates, Clinical trials, Source documents

The Necessity of Clinical Research Documentation Training Programs and the Value of Learning from Mistakes - ACRP
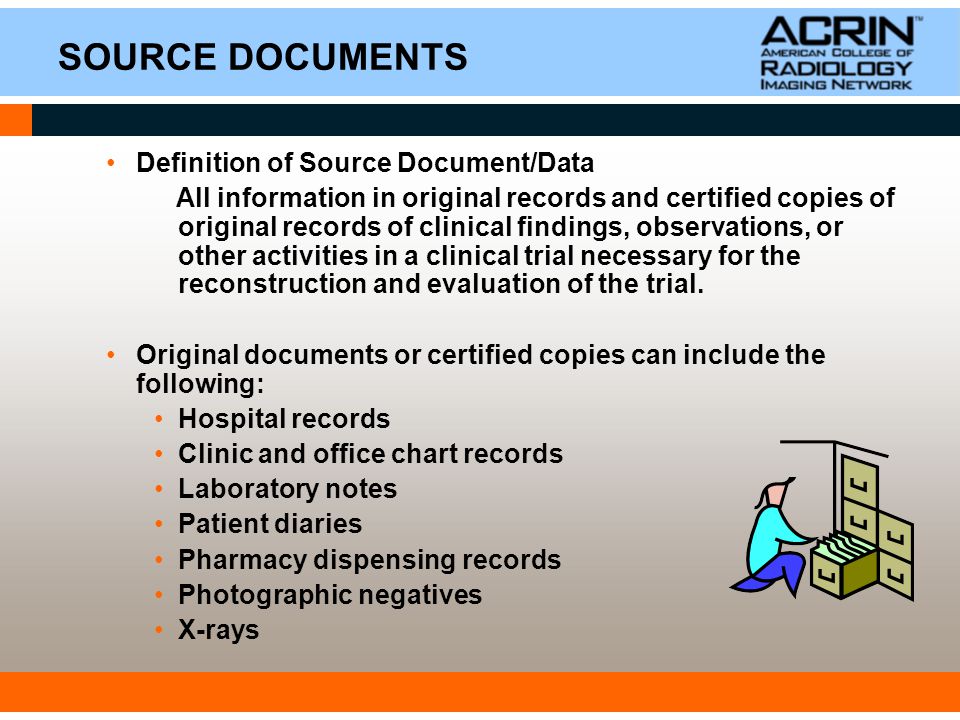
examples of source documents — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification
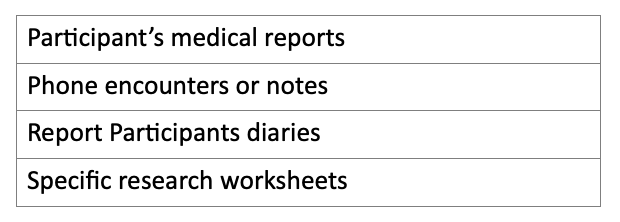
what is a source document — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification

Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca

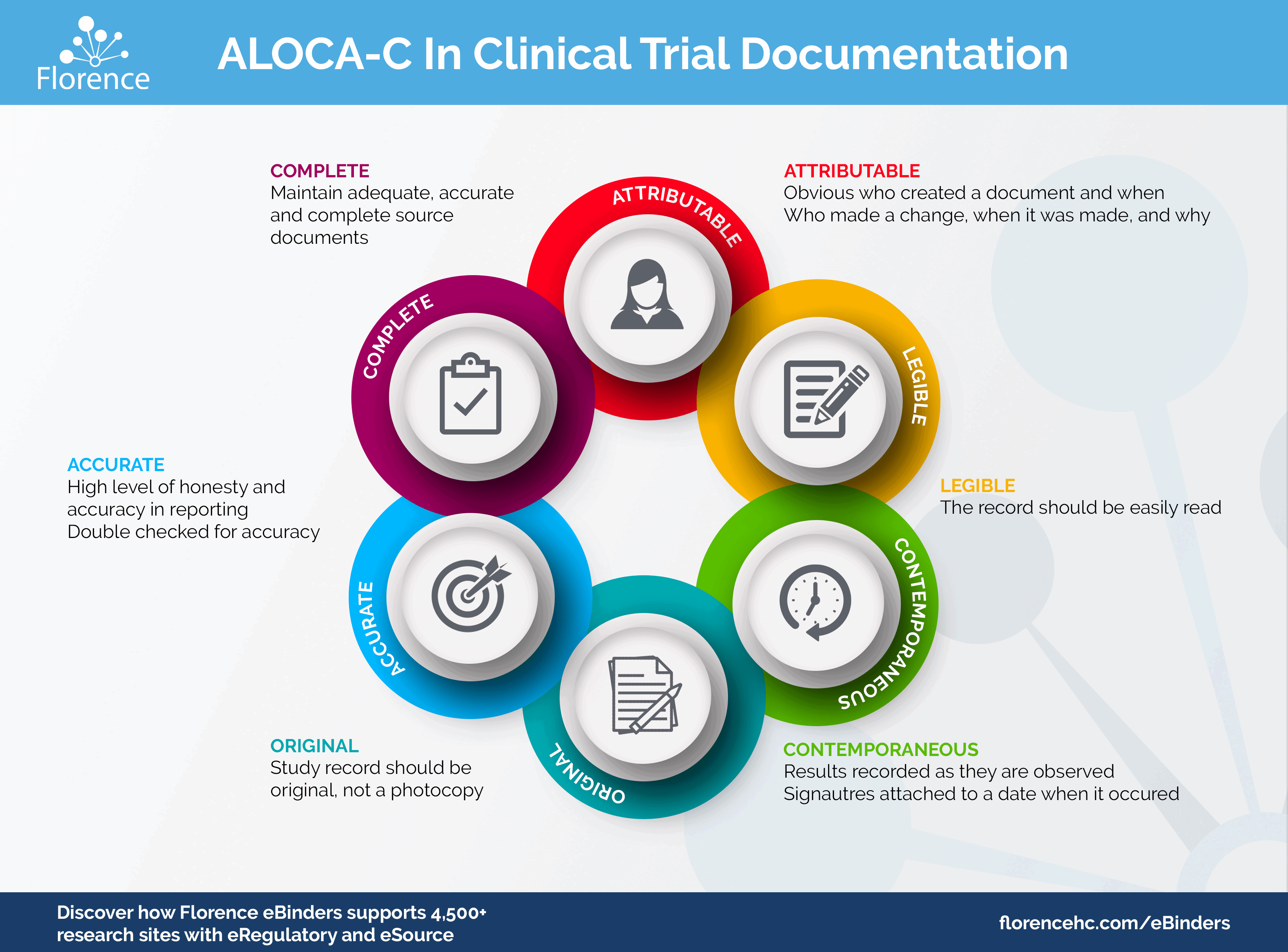
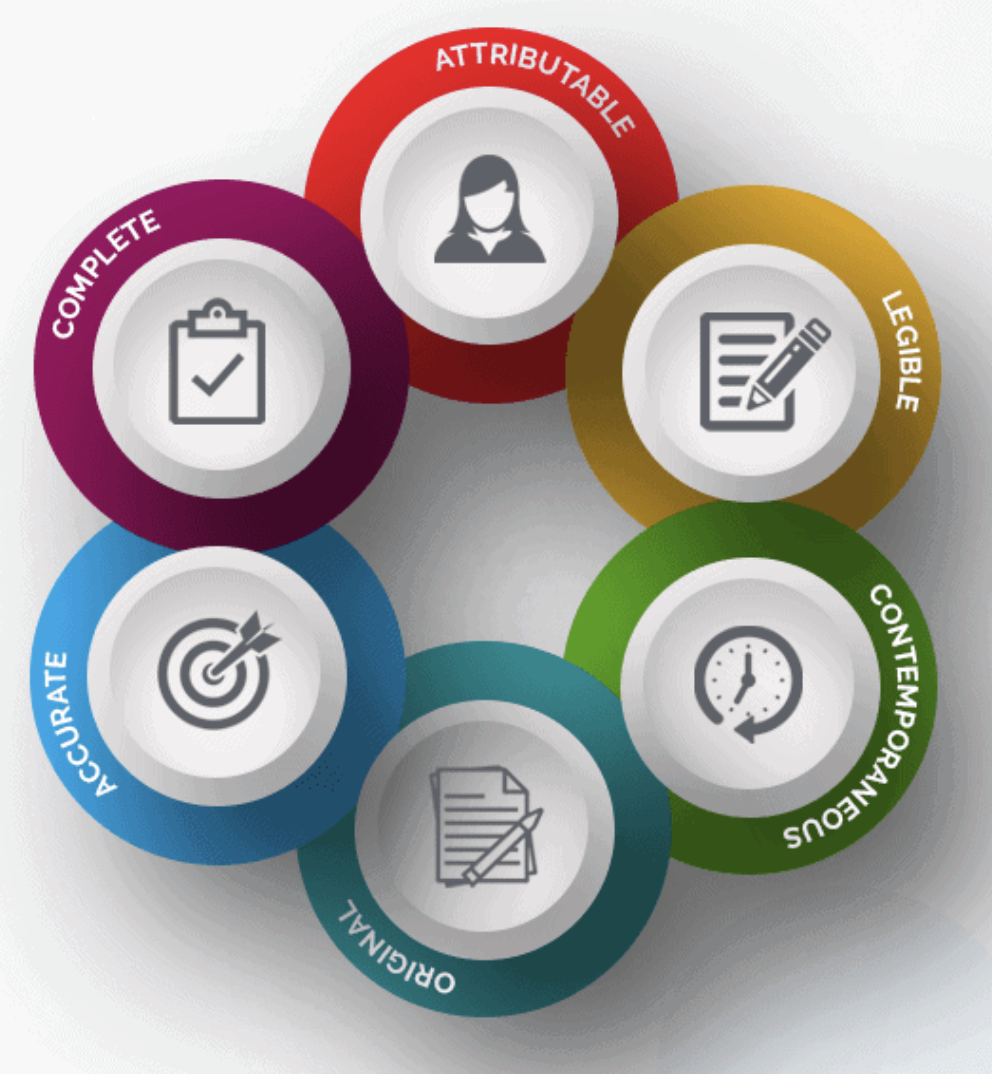
Source Documentation. Objectives: At the conclusion of this discussion, participants will be able to: –Define source document and source data –Identify. - ppt download