Melatonin reduces the anxiety (A-D) After 1 hr. of drug administration... | Download Scientific Diagram

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

FAQS and facts about clinical trials and ethical errors - Journal of Plastic, Reconstructive & Aesthetic Surgery

ClinicalTrials.gov Results Reporting, Unique Evidence, and the Role of Medical Librarians SCR CONNECTions March 19, ppt download
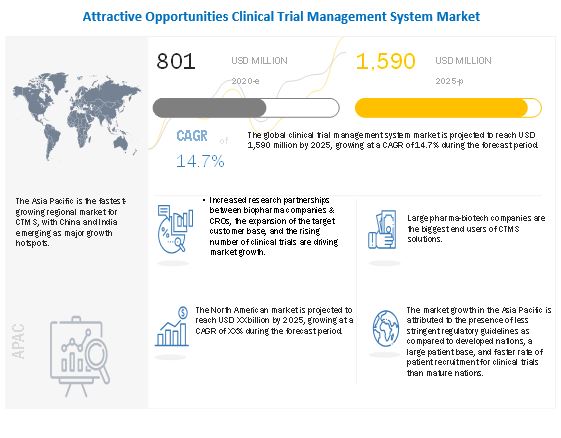
Clinical Trial Management System Market | (2022 - 2025) | Size, Share and Trends | MarketsandMarkets

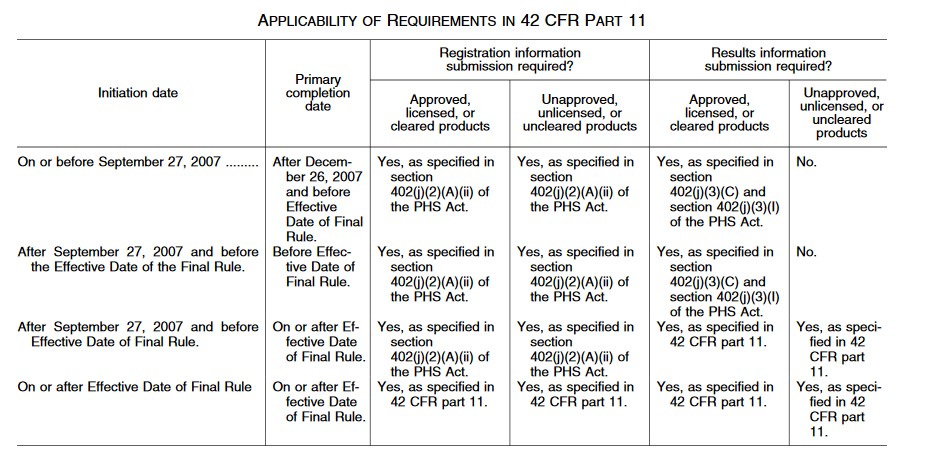
A service of the U.S. National Institutes of Health Module 1: Clinical Trials and Requirements for Registration and Results Reporting. - ppt download