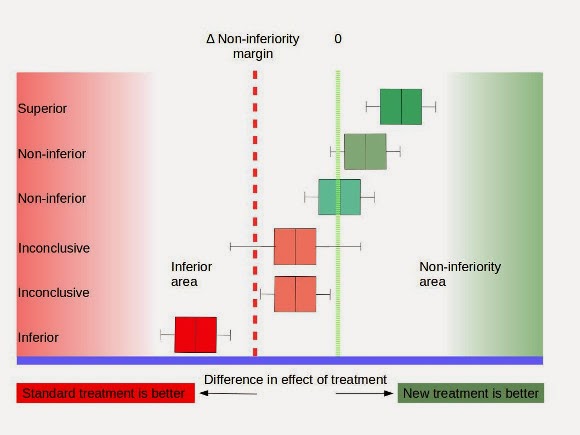
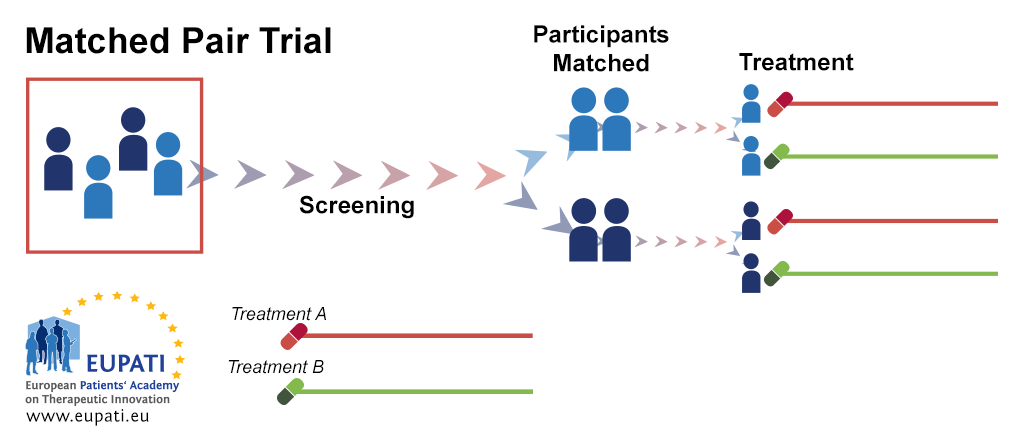
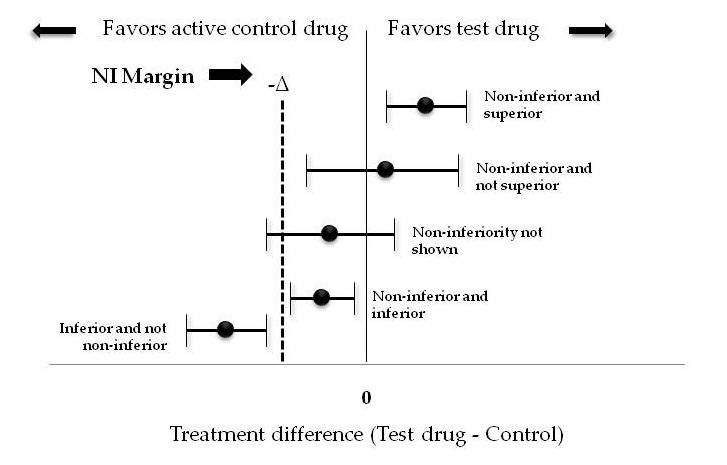
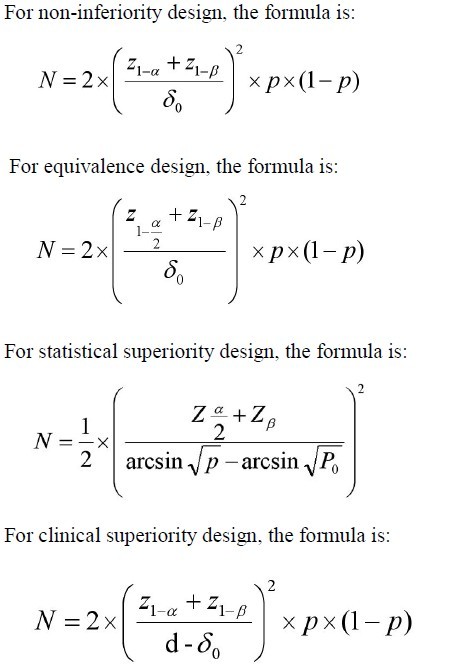
SciELO - Brasil - Non-inferiority clinical trials: importance and applications in health sciences Non-inferiority clinical trials: importance and applications in health sciences

Quality of reporting of clinical non-inferiority and equivalence randomised trials - update and extension | Trials | Full Text
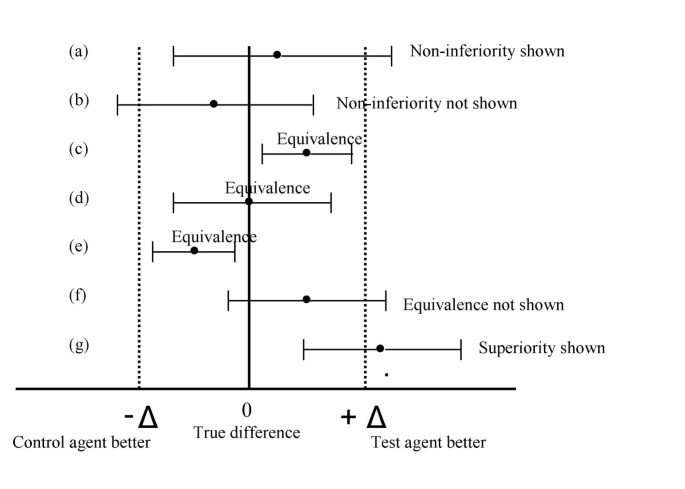
Equivalence and noninferiority trials – are they viable alternatives for registration of new drugs? (III) | Trials | Full Text
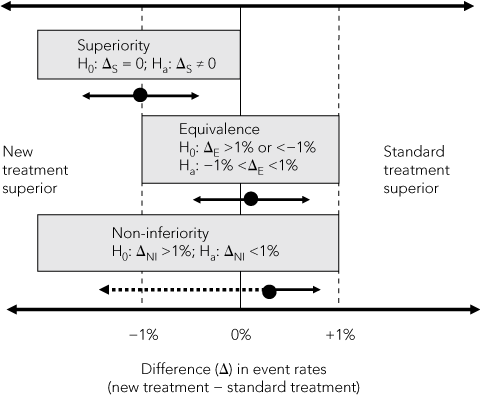
Non-inferiority trials: determining whether alternative treatments are good enough | The Medical Journal of Australia

Challenges in the Design and Interpretation of Noninferiority Trials: Insights From Recent Stent Trials - ScienceDirect
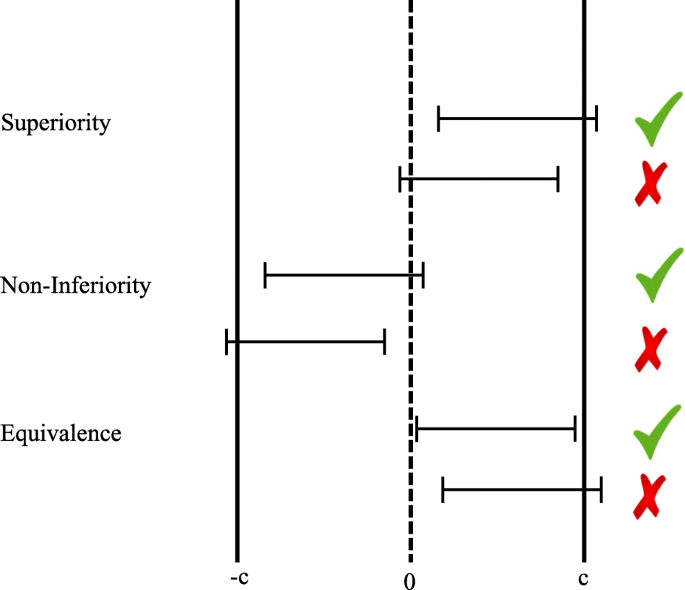
Bayes factors for superiority, non-inferiority, and equivalence designs | BMC Medical Research Methodology | Full Text

SciELO - Brasil - Estudos clínicos de não-inferioridade: fundamentos e controvérsias Estudos clínicos de não-inferioridade: fundamentos e controvérsias

What is the difference between Superiority vs. Equivalence vs. Non-inferiority in clinical trial design

How to Demonstrate Similarity by Using Noninferiority and Equivalence Statistical Testing in Radiology Research | Radiology
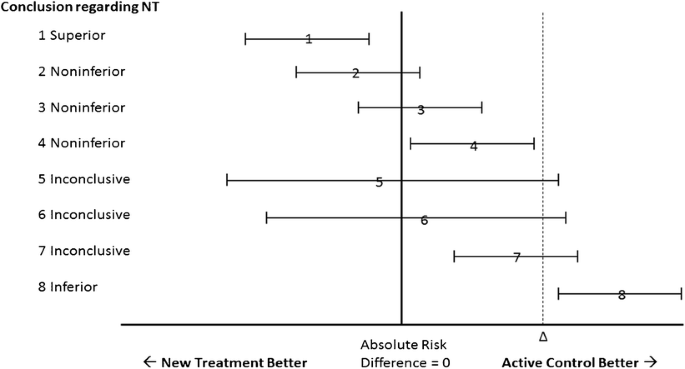
Empirical Consequences of Current Recommendations for the Design and Interpretation of Noninferiority Trials | SpringerLink