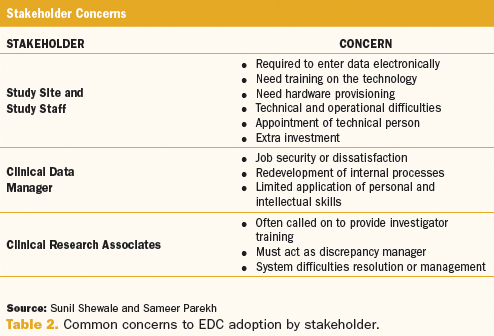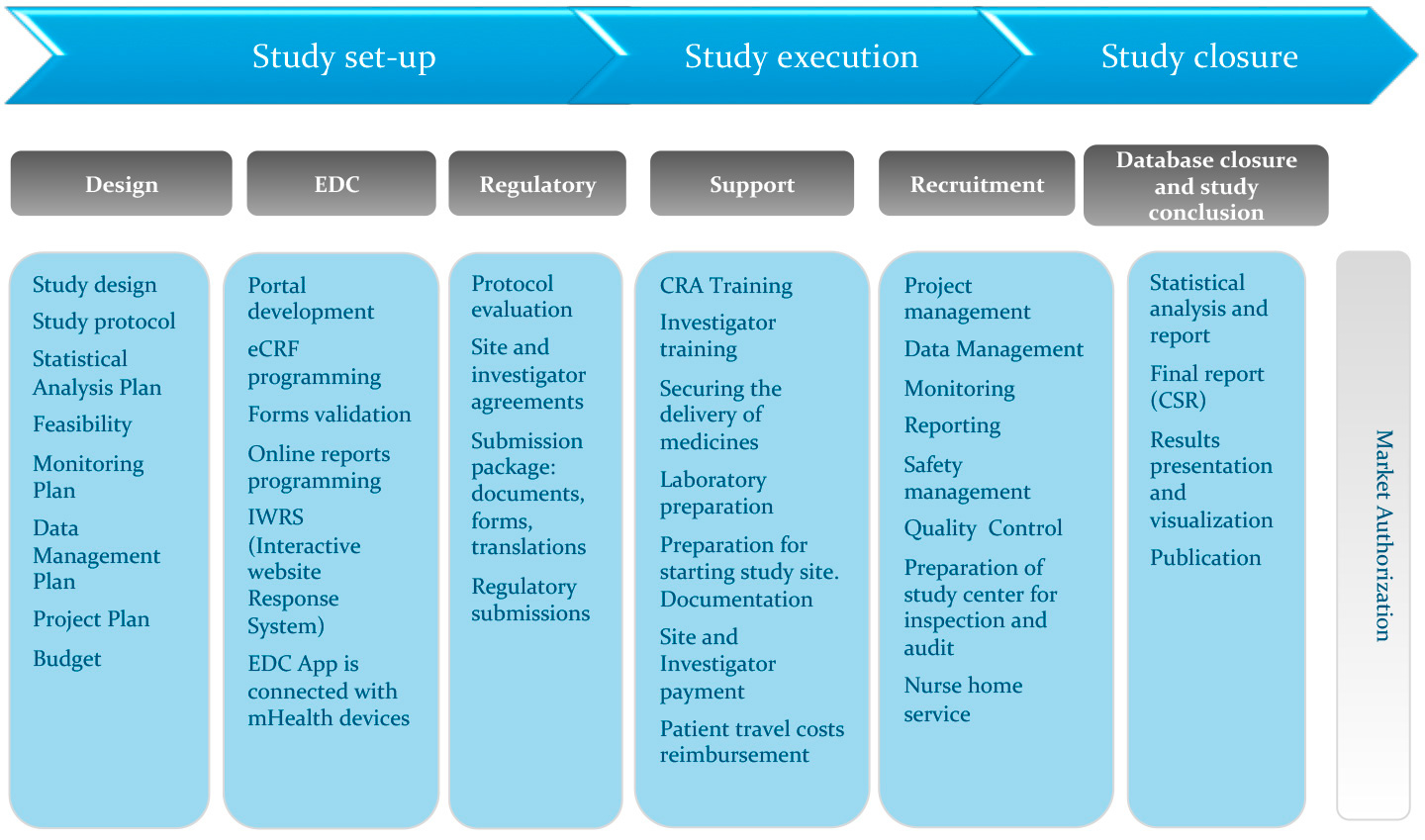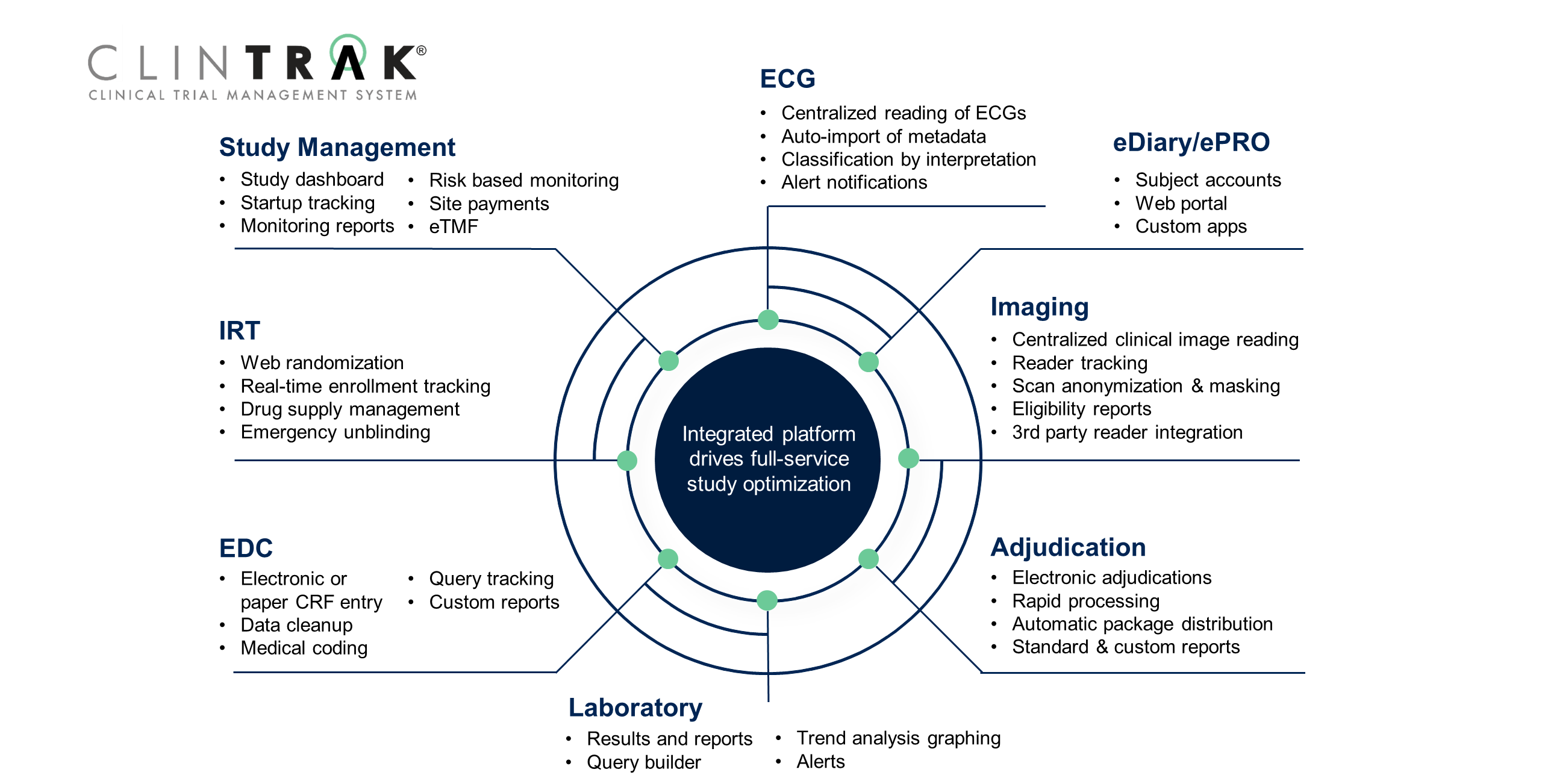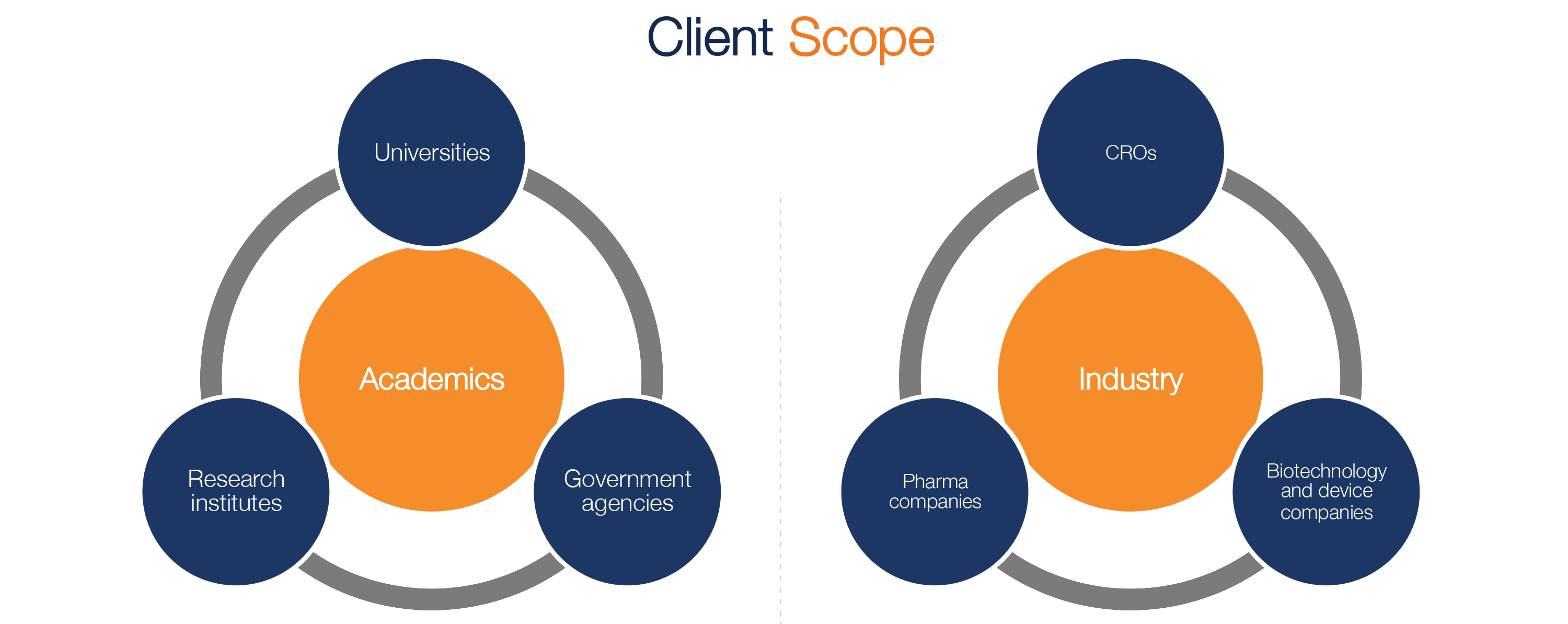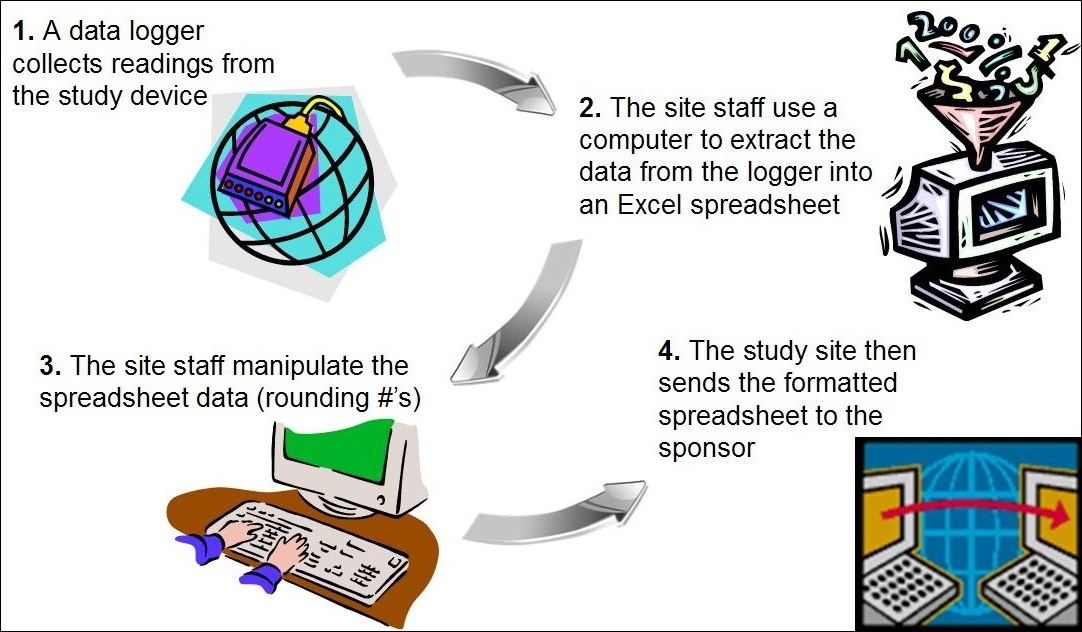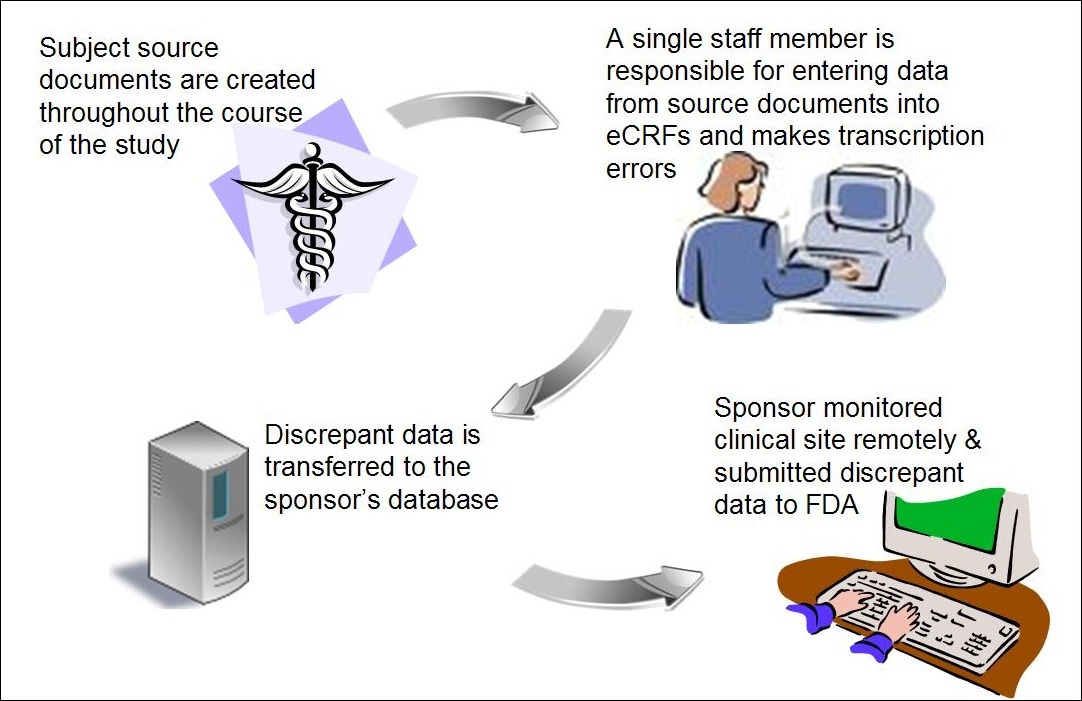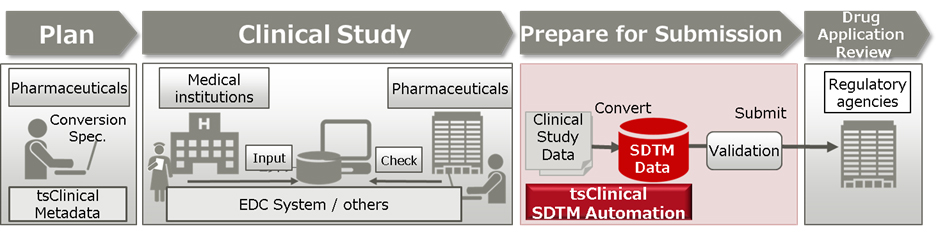
Fujitsu Launches Japan's First System for Automatically Generating Electronic Submission Data for Pharmaceuticals - Fujitsu Global
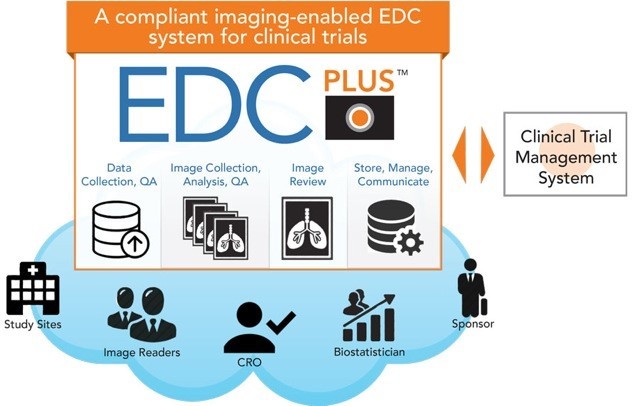
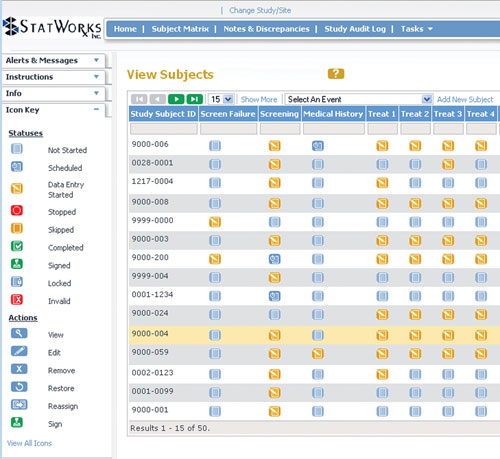
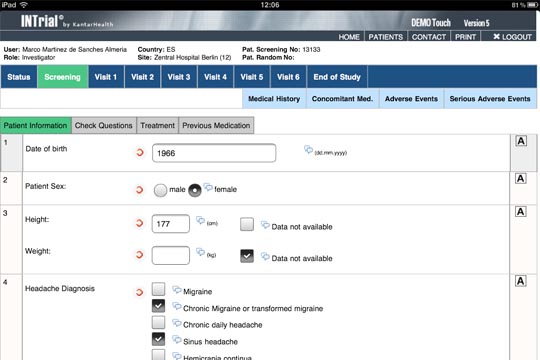
ImageIQ Releases EDCplus Technology, an Imaging-enabled Electronic Data Capture System for Clinical Trials | Business Wire