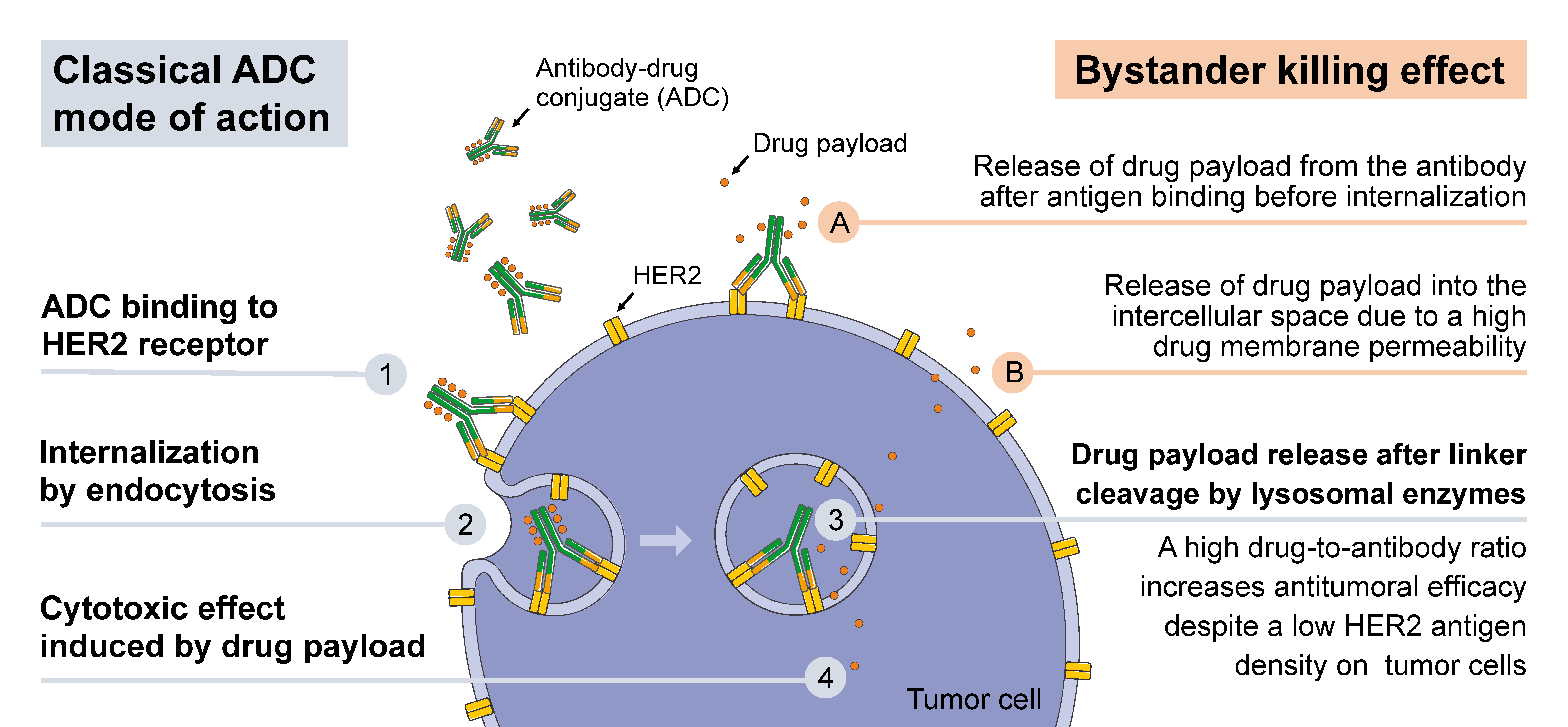
Antitumor immunity caused by combination of DS-8201a and anti-CTLA-4... | Download Scientific Diagram

Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study - The Lancet Oncology

Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial - The Lancet Oncology

Enhertu ® (fam-trastuzumab deruxtecan-nxki or DS-8201a) formula, ADC... | Download Scientific Diagram
![iPoster : [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator's choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-Breast02) iPoster : [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator's choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-Breast02)](https://assets.posterview.com/SABCS/2019/1/Jpg/f/OT1-07-04.jpg)
iPoster : [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator's choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-Breast02)

Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study - The Lancet ...

Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial - The Lancet Oncology
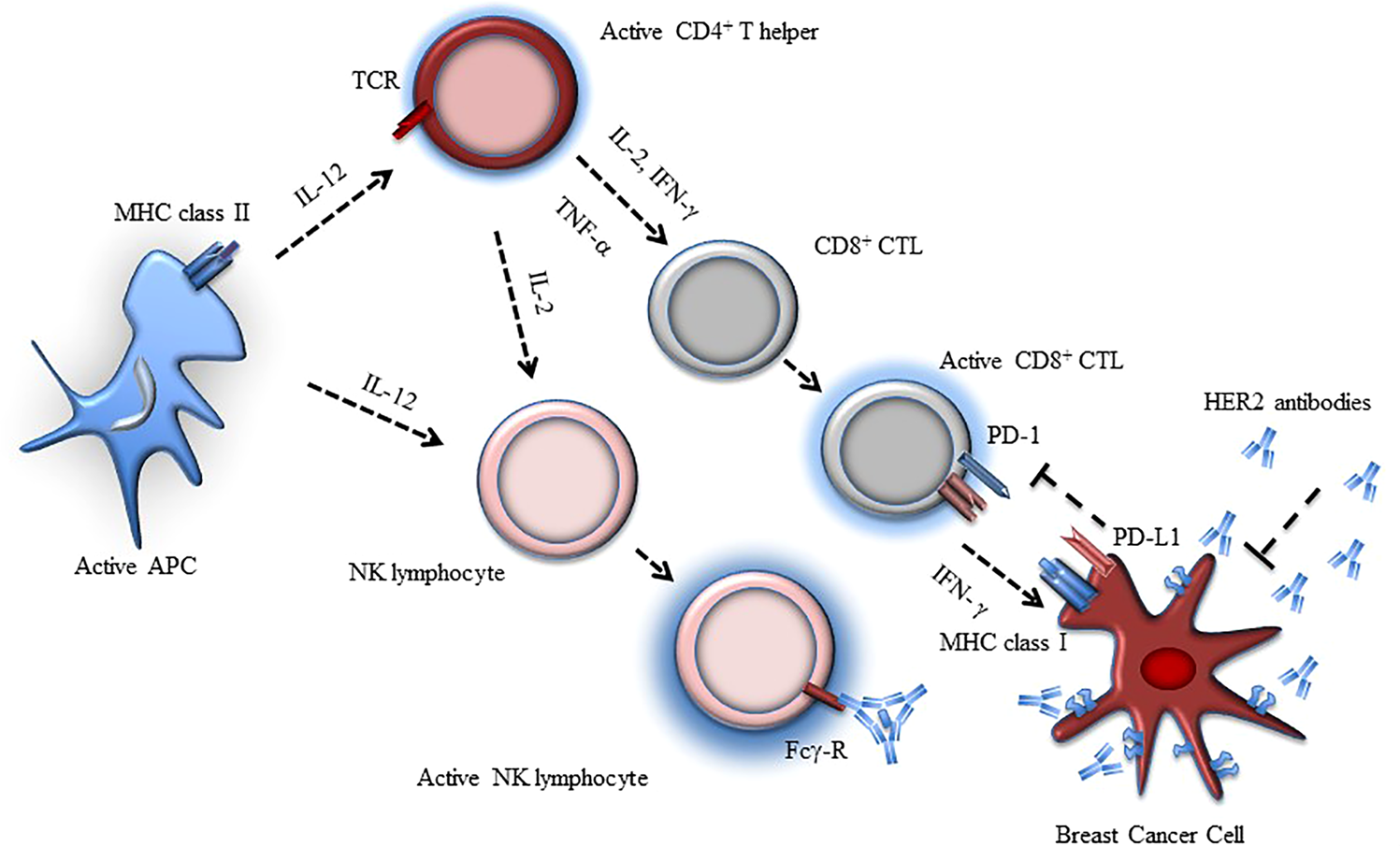
Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond | npj Breast Cancer

Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study - The Lancet Oncology

Clinical Development of New Antibody–Drug Conjugates in Breast Cancer: To Infinity and Beyond | SpringerLink

Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study - The Lancet Oncology
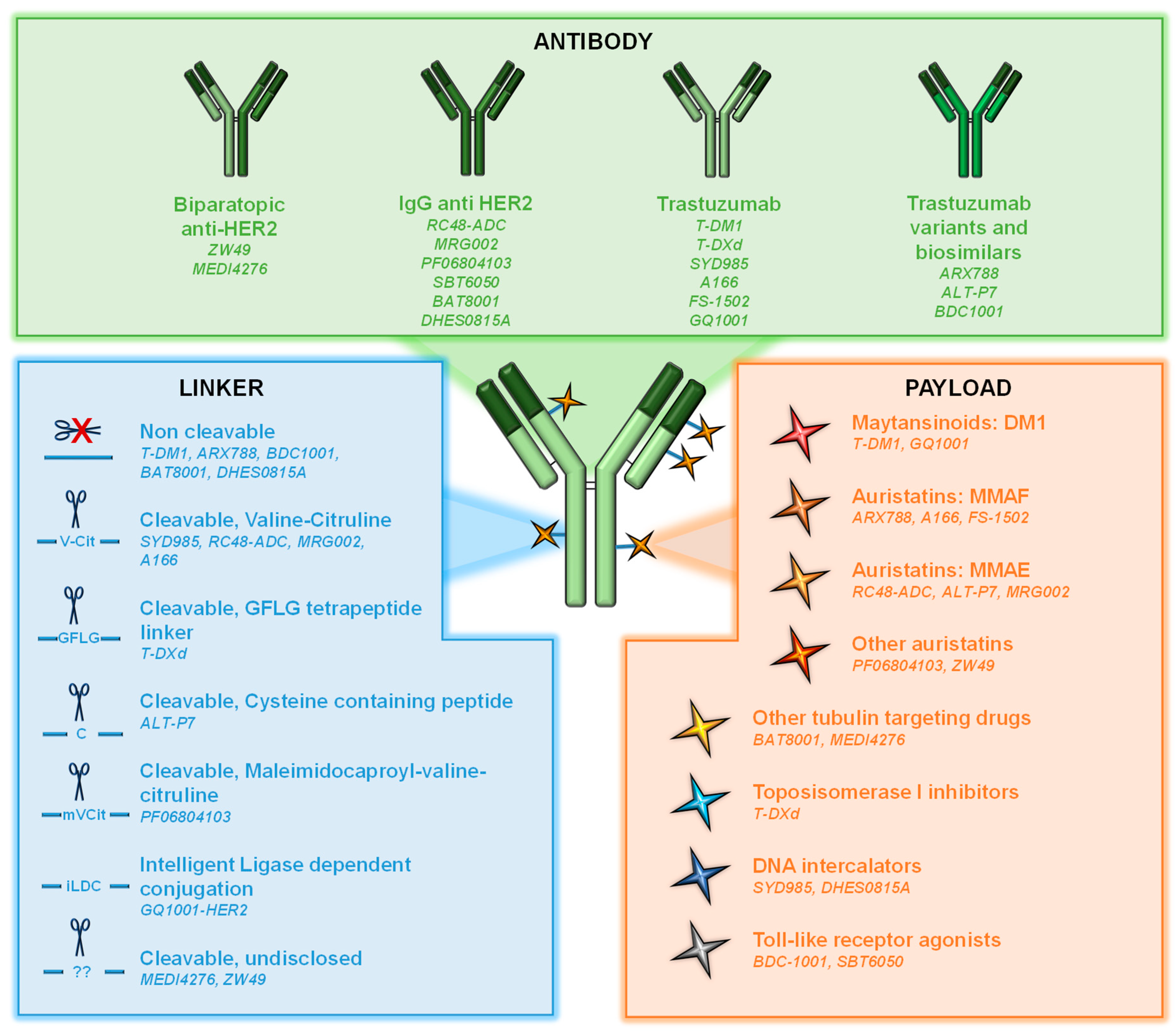
Cancers | Free Full-Text | Novel ADCs and Strategies to Overcome Resistance to Anti-HER2 ADCs | HTML
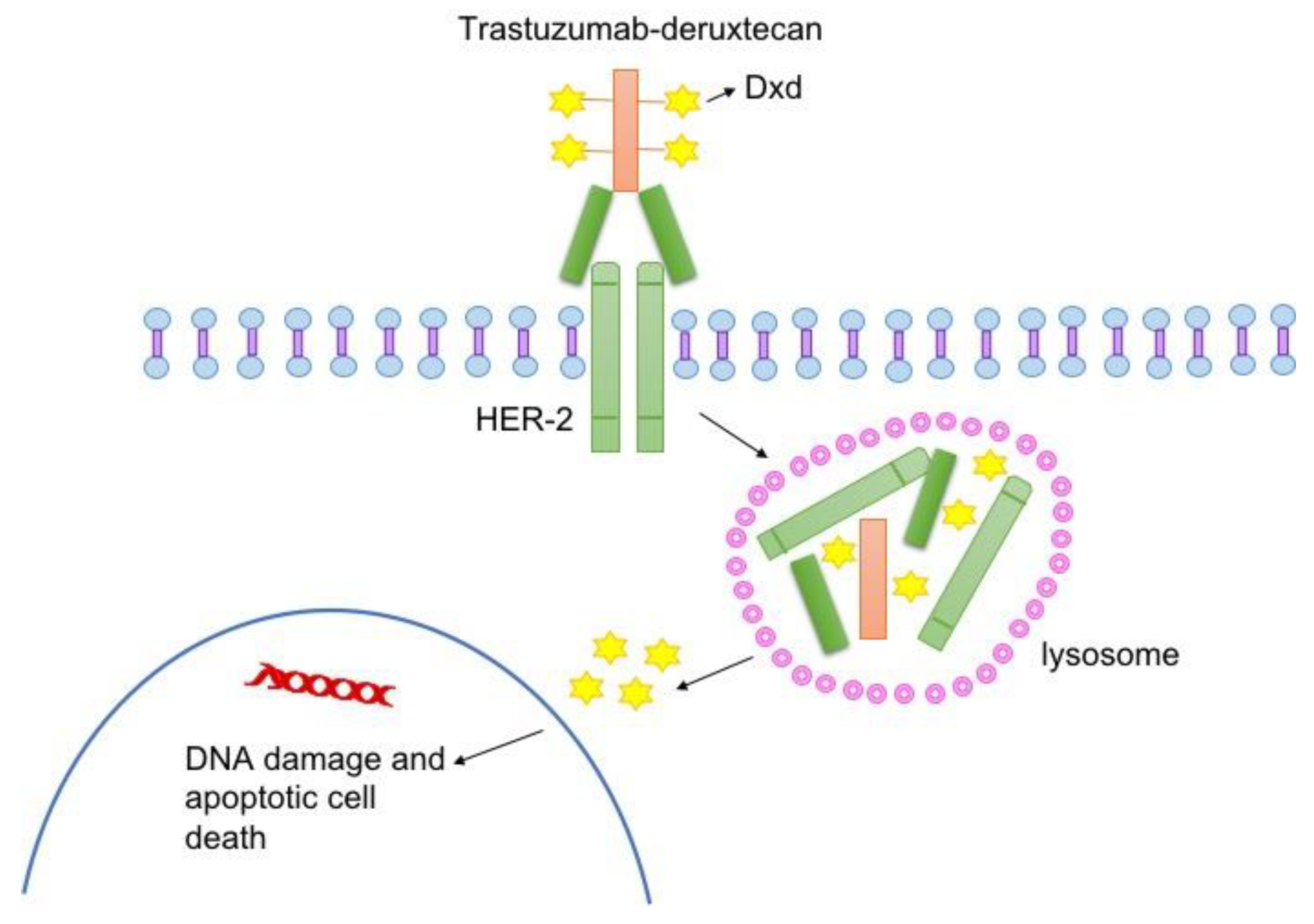
IJMS | Free Full-Text | Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors | HTML
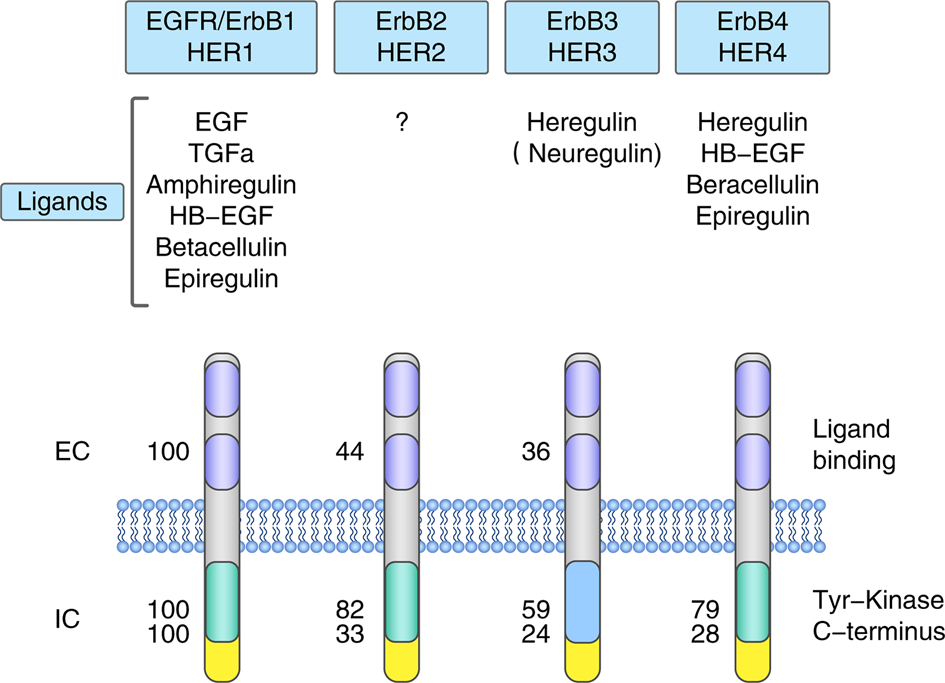
Targeted therapeutic options and future perspectives for HER2-positive breast cancer | Signal Transduction and Targeted Therapy