New regulation on clinical trials in Spain - Leon Research | CRO - Clinical Trials Spain, Italy and Portugal

PHM Conference on Twitter: "📢📢Calling all #PHMResearchers!! The submission site for #PHM21 Research Abstracts and Clinical Conundrums is now open!! The deadline for submissions is February 28 at 11:59pm EST. Submission Site:
Lessons Learned for Successful e-Study Data Submission to PMDA toward the End of Transitional Period

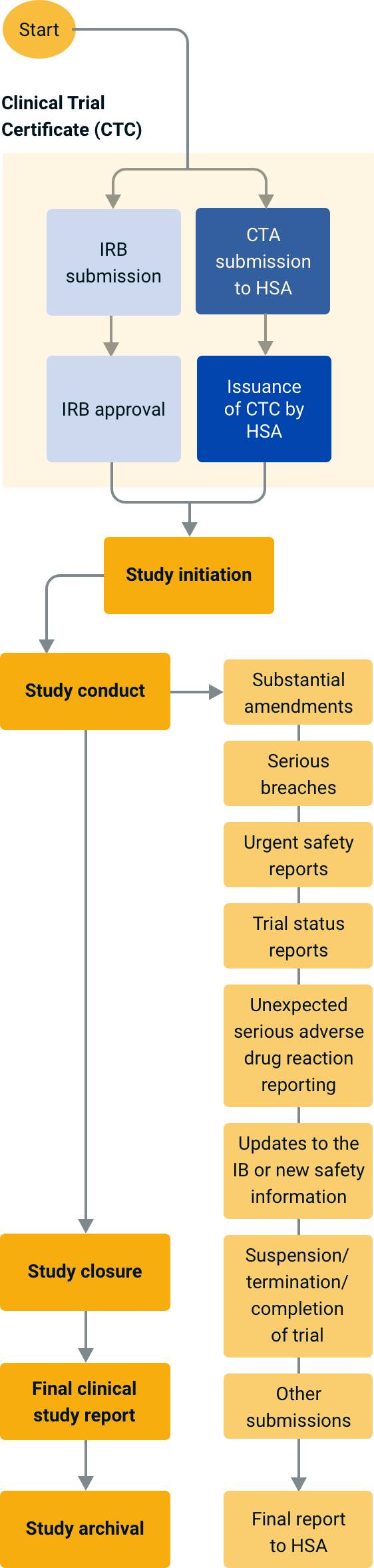
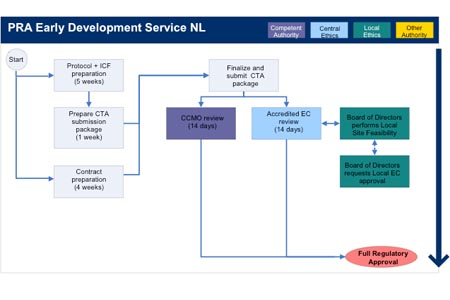
An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram

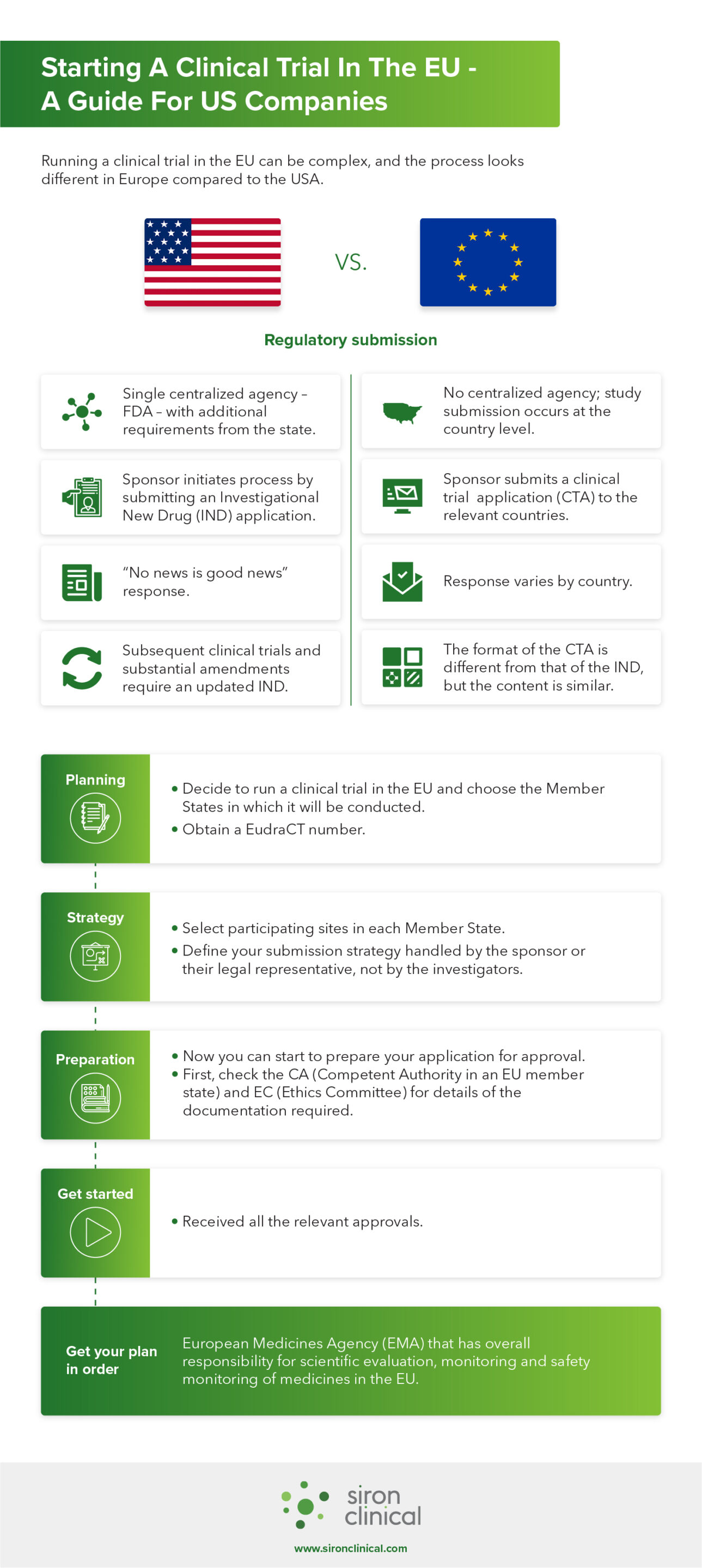
Silence Therapeutics and Mallinckrodt Announce Submission of Clinical Trial Application for SLN501 | Business Wire

Standards in the operational management of clinical trials and their... | Download Scientific Diagram

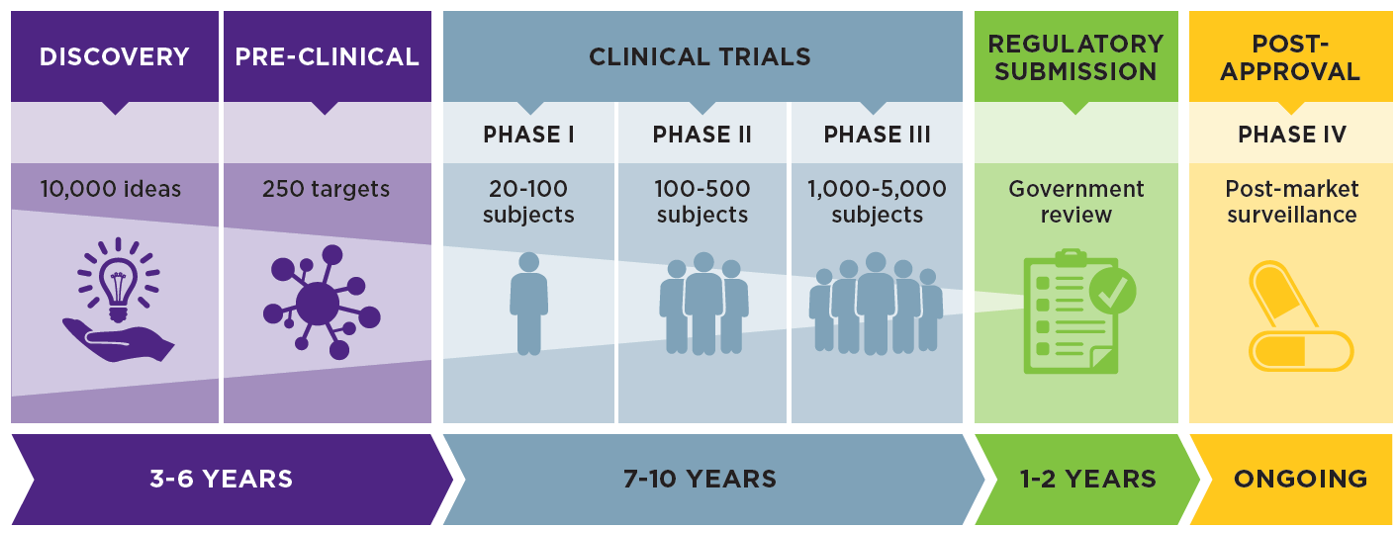
Case Study 43: Using foreign Clinical Data for the Submission of IND in USA - Global Regulatory Partners, Inc.

Frontiers | EUPATI and Patients in Medicines Research and Development: Guidance for Patient Involvement in Ethical Review of Clinical Trials | Medicine